New ISOPURE SERENITY Premium Monofocal hydrophobic IOL is engineered to provide cataract patients with a consistent range of vision, from excellent distance vision1 to good intermediate vision2 in all lighting conditions, without compromising quality of vision3 or causing any visual disturbances.3,4 Featuring patented, non-diffractive ISOFOCAL optic technology and the unique double C-loop POD platform for IOL stability.
The Second Generation
from Our ISOPURE Family
Find Your Serenity
Extended Range of Vision
Compared to a Monofocal IOL, ISOPURE SERENITY:
- Increases depth of focus by approximately 50% 5
- With only 12% decrease in maximum MTF 5
This is equivalent to approximately 1.0D of extended depth of focus.

Uncompromised Quality of Vision
ISOFOCAL technology uses non-diffractive optics, eliminating rings or steps which impact the quality of the patient’s vision.
The ISOPURE SERENITY optic has been proven to exhibit photic phenomena comparable to that of a standard monofocal IOL.3
Simplified for the Surgeon
The ISOPURE SERENITY optic features a non-diffractive design, simplifying the discussions for patients who are not candidates for diffractive technology but still desire an extended range of vision and reduced dependence on spectacles for intermediate vision.
RANGE OF VISION UNCOMPROMISED
QUALITY OF VISION
THE RIGHT BALANCE
Effect of Spherical Aberration
Extended Range Of Vision
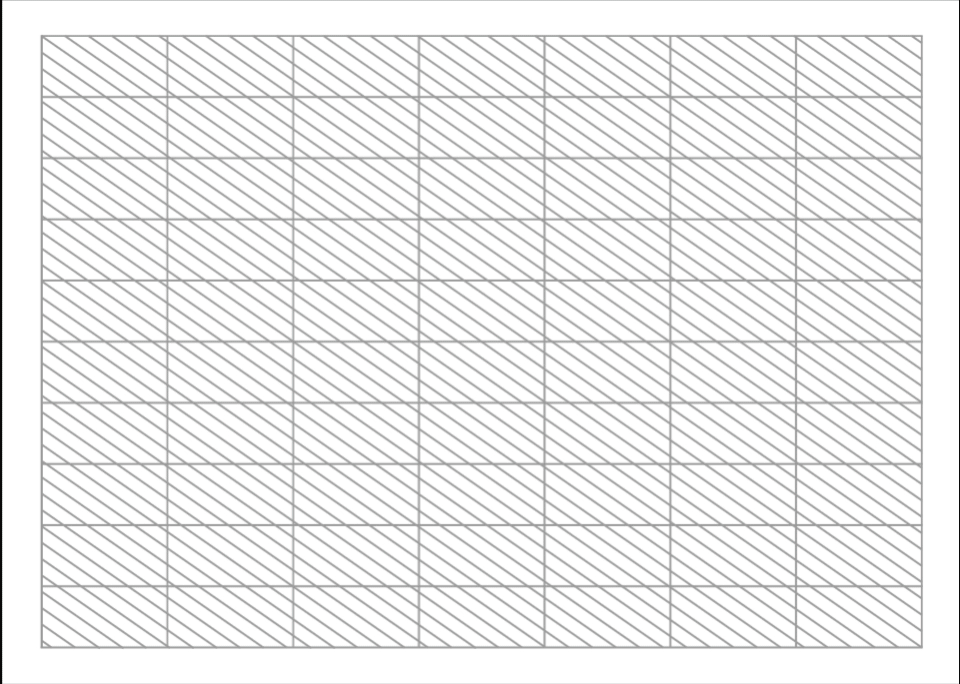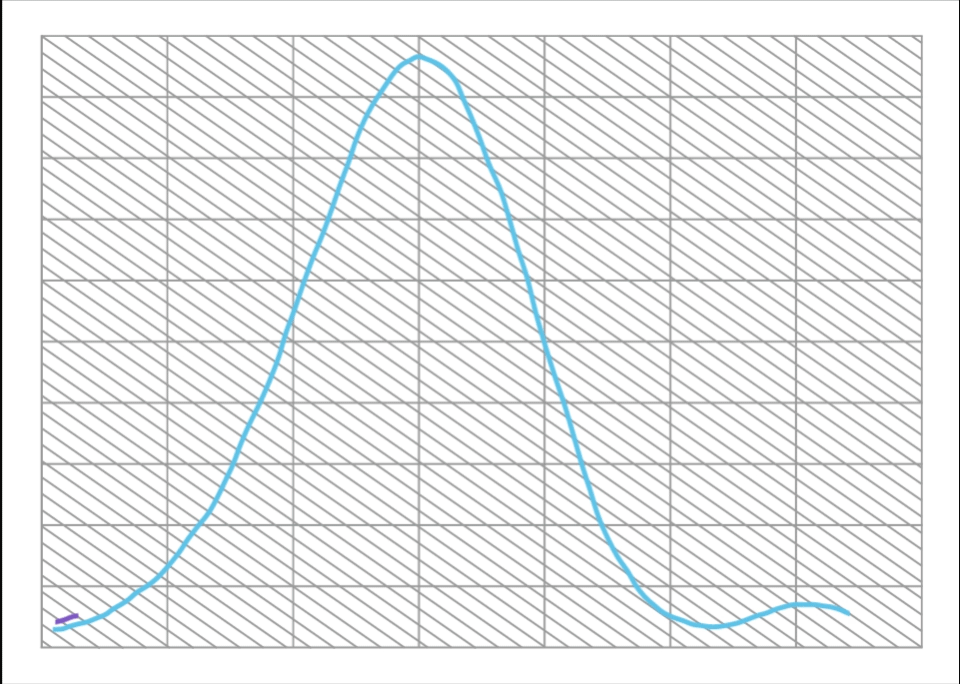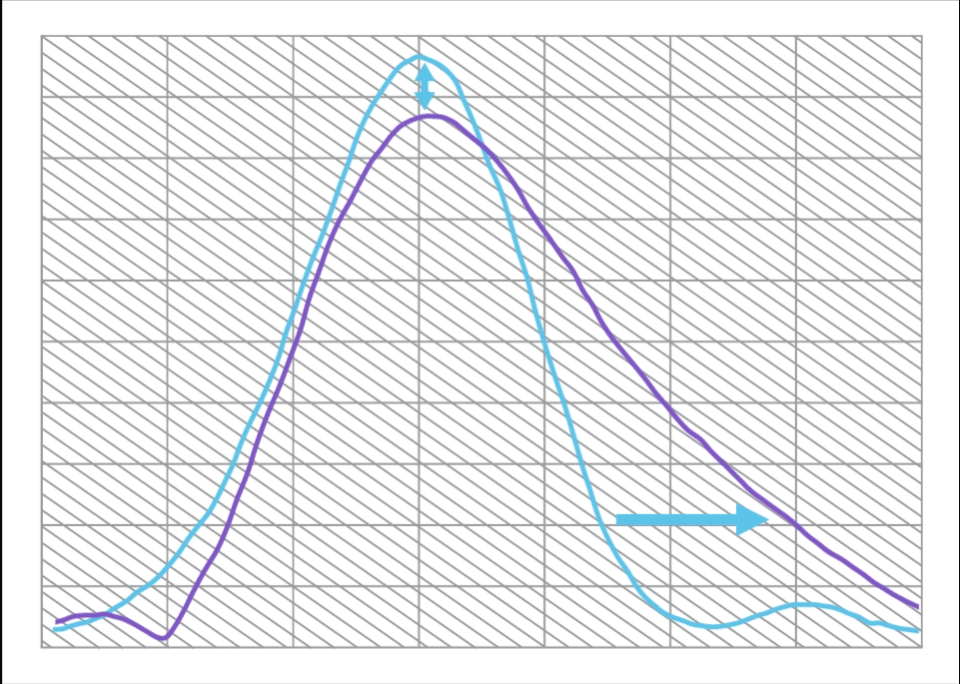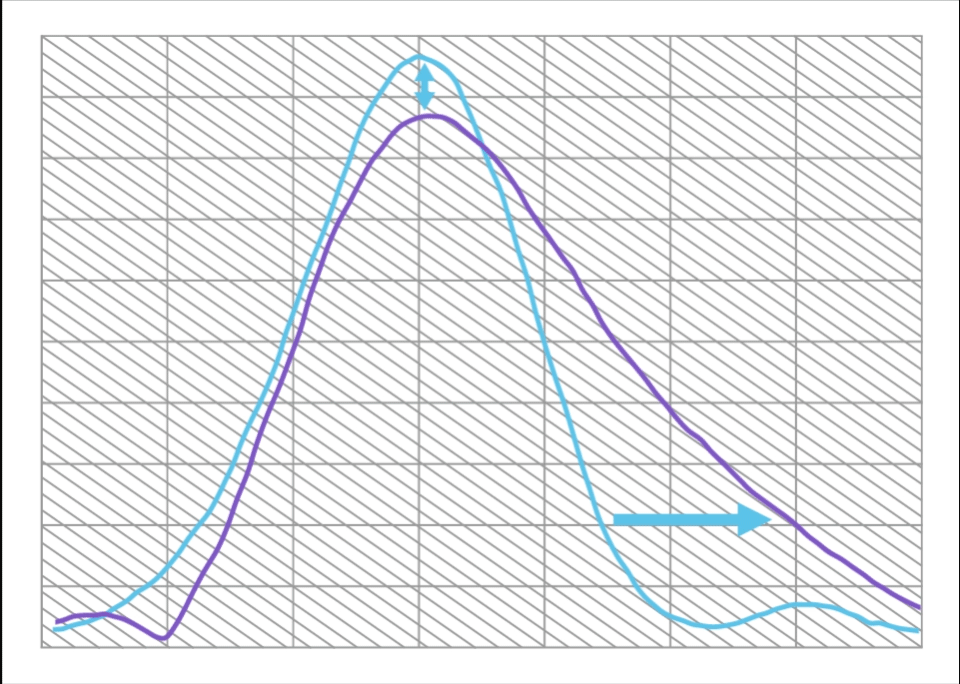

Compared to a Monofocal IOL, ISOPURE SERENITY:
- Increases range of vision by approximately 50%
- With only 12% decrease in maximum MTF
This is equivalent to approximately 1.0D of extended range of vision.
PATIENT OUTCOMES
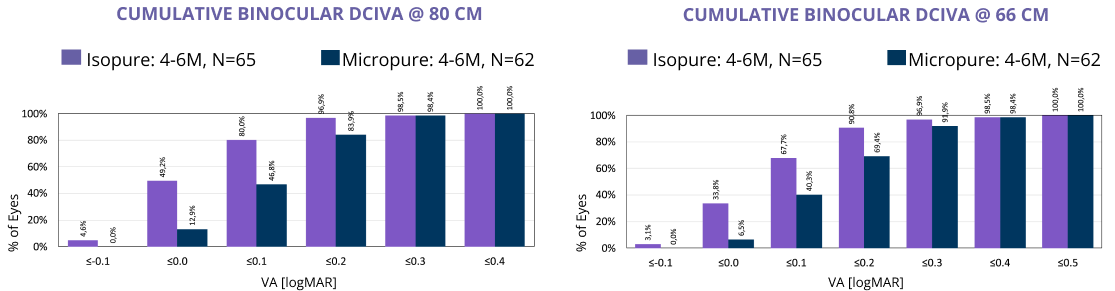
Clinical studies2 have repeatedly shown an increased range of vision up to 66cm using ISOPURE optic technology.
- 80% achieve VA 0.1LogMAR at 80cm
- 60% achieve VA 0.1LogMAR at 66cm
Extended Range Of Vision and Customised Spherical Aberration
ISOPURE is the only range of premium monofocal IOLs that utilizes ISOFOCAL technology, incorporating spherical aberration across the full optic diameter and on both the anterior and posterior optical surfaces.
- ISOFOCAL technology is unique, patented to BVI.
- ISOPURE are the only lenses to progressively adjust the spherical aberration value across the entire optical surface.
ISOFOCAL technology is designed to further adjust the value of spherical aberration per individual lens power; therefore, adapting the full optical system to fine tune the patient’s extended range of vision.

Please note that these drawings are for illustrative purposes only and serve as a general representation of the Intraocular Lens (IOL) design
In this large, prospective, randomized study, ISOPURE optic technology consistently performed better in unaided intermediate vision compared to a standard monofocal (80 cm and 66 cm).3
Uncompromised Quality of Vision
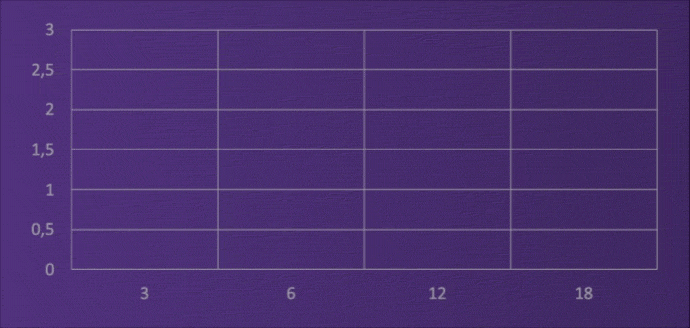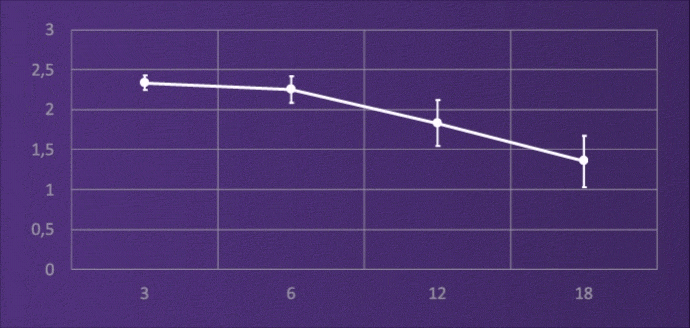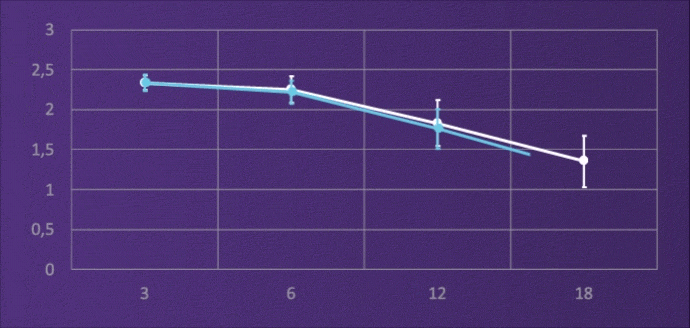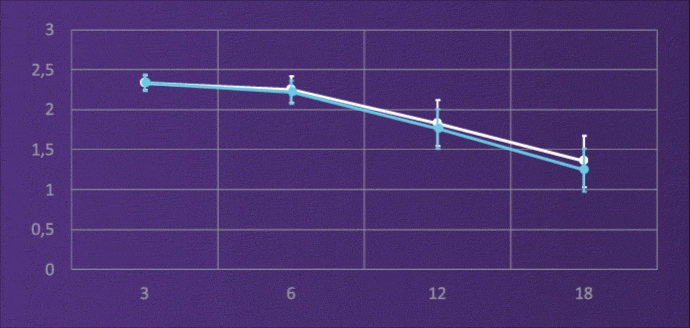
Like a monofocal IOL, the ISOPURE SERENITY optic uses all the available light energy to extend the range of focus.
It does not lose light energy through diffraction like multifocal IOLs, and through this design it maintains contrast sensitivity comparable to a monofocal.3
CONTRAST SENSITIVITY, BINOCULAR
4-6M, PHOTOPIC

Advanced IOL Solutions to Meet the Diverse Needs of Surgeons and Patients
References
1.Stodulka P, Slovak M. Visual performance of a polynomial extended depth of focus intraocular lens. Open Journal of Ophthalmology 2021;11:214-228.
2. Bernabeu-Arias G, Beckers S, Rincón-Rosales JL, Tañá-Rivero P, Bilbao-Calabuig R. Visual Performance at Different Distances After Implantation of an Isofocal Optic Design Intraocular Lens. J Refract Surg. 2023 Mar;39(3):150-157.
3. Ang RET, Stodulka P, Poyales F. Prospective Randomized Single-Masked Study of Bilateral Isofocal Optic-Design or Monofocal Intraocular Lenses. Clinical Ophthalmology. 2023.
4. Tomagova N, Elahi S, Vandekerckhove K. Clinical Outcomes of a New Non-Diffractive Extended Depth-of-Focus Intraocular Lens Targeted for Mini-Monovision. Clin Ophthalmol. 2023 Mar 25;17:981-990.
5. BVI data held on file.
Additional Information
Not all products or offerings are approved or offered in every market. Approved labelling and instructions may vary from one country to another. Contact your local distributor or BVI (Contact us – BVI Medical) for worldwide product information.
This product is not approved by the FDA for use or distribution in the United States of America.
Instructions for Use
To access labeling documents for BVI Medical Device products, please access our IFUs website.
Clinical Data
To consult our extensive collection of clinical resources on BVI’s products and technologies, please access our Bibliography website.






 International
International
 Brasil
Brasil Danmark
Danmark Deutschland
Deutschland España
España France
France Italia
Italia 日本
日本 Nederland
Nederland Norge
Norge Suomi
Suomi Sverige
Sverige United States
United States United Kingdom
United Kingdom




 Contact us
Contact us