New ISOPURE SERENITY TORIC Premium Monofocal hydrophobic IOL is engineered to provide cataract patients with a consistent range of vision, from excellent distance vision1 to good intermediate vision2 in all lighting conditions, without compromising quality of vision3 or causing any visual disturbances.3,4
Featuring patented, non-diffractive ISOFOCAL optic technology and our ground-breaking, double C-loop POD platform — specifically designed for the improved stability required by toric IOLs for long-term, accurate astigmatism correction.
The Second Generation
from Our ISOPURE Family
Find Your Serenity
POD Platform for Clinically Proven Rotational Stability
From 1 hour to 3 months postoperatively
1.22°
of average rotation6 with the PODEYE Toric lens
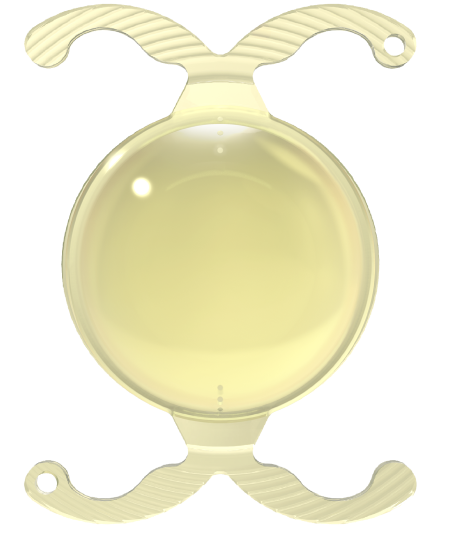
The BVI POD Platform:
Specifically Engineered for Toric IOL Stability
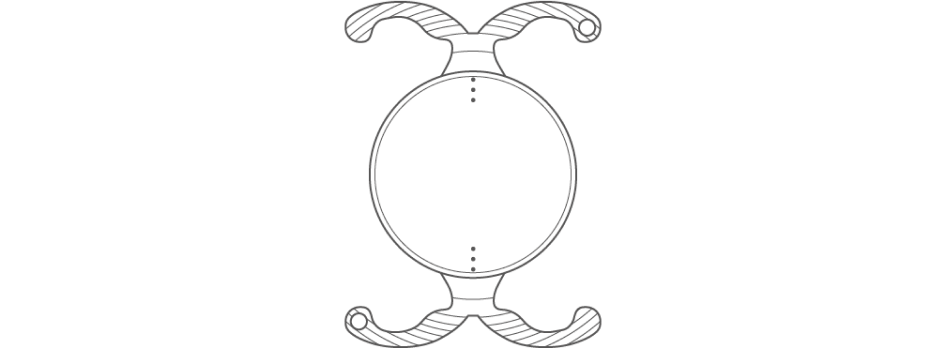

Stability for Long-term, Accurate Astigmatism Correction
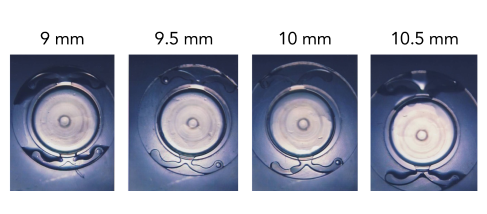
POD Platform for Clinically Proven Rotational Stability
From 1 hour to 3 months
1.22°
postoperatively of average rotation6
with the PODEYE Toric lens
Clinically Proven Design
POD platform with
Over 13 years
of IOL experience, providing reliability
in terms of clinical outcomes11

Maneuverability for Ease of Use
Easy Control During the Procedure7
Rotation to align the IOL cylinder, either clockwise OR counter-clockwise.8

Whereas classic C-loop IOLs can only be rotated clockwise and require additional steps in case of misalignment.8
Unique RidgeTech technology reduces the risk12 of sticky haptics on the optics during and after injection.

Accuracy for More Predictable Results
Toric IOL selection with built in
Abulafia-Koch
(AK) Formula
Toric Calculator9 with AK formula delivers
94%
of eyes with less than 0.75D of absolute predicted residual astigmatism10
Our toric calculator has been developed to compensate the posterior corneal astigmatism effect by improving the prediction of postoperative astigmatic patient outcomes.13
Advanced IOL Solutions to Meet the Diverse Needs of Surgeons and Patients
References
1.Stodulka P, Slovak M. Visual performance of a polynomial extended depth of focus intraocular lens. Open Journal of Ophthalmology 2021;11:214-228.
2. Bernabeu-Arias G, Beckers S, Rincón-Rosales JL, Tañá-Rivero P, Bilbao-Calabuig R. Visual Performance at Different Distances After Implantation of an Isofocal Optic Design Intraocular Lens. J Refract Surg. 2023 Mar;39(3):150-157.
3. Ang RET, Stodulka P, Poyales F. Prospective Randomized Single-Masked Study of Bilateral Isofocal Optic-Design or Monofocal Intraocular Lenses. Clinical Ophthalmology. 2023.
4. Tomagova N, Elahi S, Vandekerckhove K. Clinical Outcomes of a New Non-Diffractive Extended Depth-of-Focus Intraocular Lens Targeted for Mini-Monovision. Clin Ophthalmol. 2023 Mar 25;17:981-990.
5. BVI data held on file.
6. Robert Edward T Ang, Pedro Tañá-Rivero, Francisco Pastor-Pascual, Pavel Stodulka, Manfred Tetz, Isaak Fischinger. Visual and Refractive Outcomes After Bilateral Implantation of a Biconvex Aspheric Toric Monofocal Intraocular with a Double C-Loop Haptic Design. Clinical Ophthalmology 2023:17 2765–2776.
7. Ang RET. “PODEYE Toric Clinical Outcomes.”Presentation, BVI Advisory Board meeting, Boston 2024.
8. Torio et al. Comparison of the Rotational Stability of Different Toric Intraocular Lens Implants. Philipp J Ophthalmol 2014;39:67-72.
9.
10. Insert CRSToday Europe, January 2018.
11. Periodic Clinical Evaluation Report.
12. Physiol Report 002, 9 nov 2012.
13. Abulafia A, Koch DD, J Cataract Refract Surg 2016, 42(5):663-671.
Additional Information
Not all products or offerings are approved or offered in every market. Approved labelling and instructions may vary from one country to another. Contact your local distributor or BVI (Contact us – BVI Medical) for worldwide product information.
This product is not approved by the FDA for use or distribution in the United States of America.
Instructions for Use
To access labeling documents for BVI Medical Device products, please access our IFUs website.
Clinical Data
To consult our extensive collection of clinical resources on BVI’s products and technologies, please access our Bibliography website.






 International
International
 Brasil
Brasil Danmark
Danmark Deutschland
Deutschland España
España France
France Italia
Italia 日本
日本 Nederland
Nederland Norge
Norge Suomi
Suomi Sverige
Sverige United States
United States United Kingdom
United Kingdom




 Contact us
Contact us